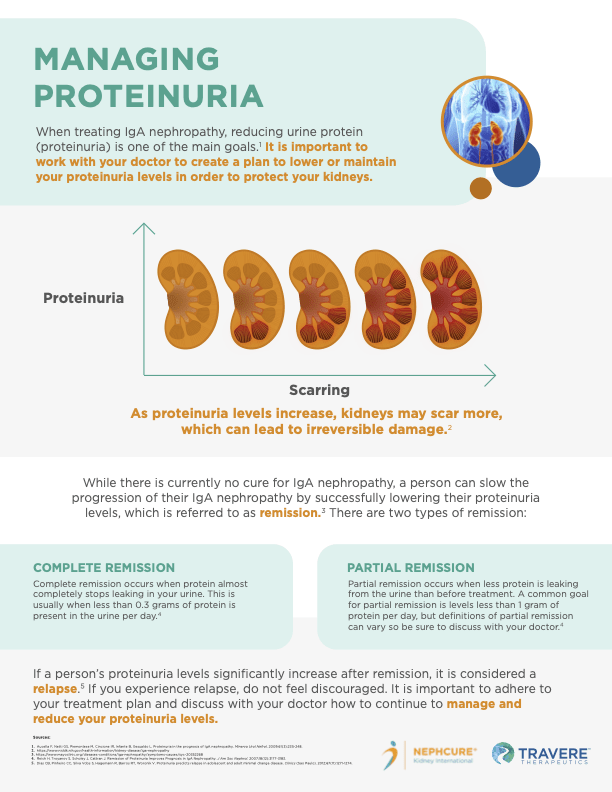
Reflecting Organization’s Focus on Rare Kidney Disease
 KING OF PRUSSIA, PA (March 31, 2023) — NephCure, formerly NephCure Kidney International, announced Friday a new visual and written brand more distinctly centered around the rare kidney disease (RKD) community they serve. With nearly a year of research and development behind it, this new brand more accurately reflects the nonprofit’s unique position in the RKD space, as well as the explosive growth and transformation the RKD field has seen over the past several years.
KING OF PRUSSIA, PA (March 31, 2023) — NephCure, formerly NephCure Kidney International, announced Friday a new visual and written brand more distinctly centered around the rare kidney disease (RKD) community they serve. With nearly a year of research and development behind it, this new brand more accurately reflects the nonprofit’s unique position in the RKD space, as well as the explosive growth and transformation the RKD field has seen over the past several years.
With this incredible growth, NephCure has entered a new era, and now has the opportunity to reach and support more people affected by RKD across the world. Through robust research, the patient advocacy organization discovered how critical it is to distinguish their brand, allowing those who may be new to the community to understand NephCure’s direct connection to RKD and collective goal to find a cure.
“We are extremely proud to unveil our new branding. It not only represents the wave of innovation in the rare kidney disease space that NephCure, researchers, industry partners, and other key stakeholders have helped cultivate together, but also encapsulates the incredibly special community fostered at NephCure,” said NephCure’s CEO, Josh Tarnoff. “We are on the cusp of monumental breakthroughs in rare kidney disease research. New treatments and innovations are upon us, and our updated branding now conveys this cutting-edge shift taking place.”
Unique among the major kidney organizations, NephCure is squarely focused on nephrotic syndrome, FSGS, IgAN, and other rare, protein-spilling kidney diseases. NephCure acts as the connector amongst patients and families, healthcare providers, government agencies, industry partners, and other key stakeholders, while also guiding patients to the best care and treatment options possible.
“We’ve listened to you — our valued community of patients, caregivers, volunteers, researchers, and other key supporters — through extensive rounds of interviews, surveys, and analysis to ensure that everyone who is a part of our community feels represented, understood, and, ultimately, inspired for progress,” said Kylie Karley, NephCure’s Marketing & Communications Director.
Initial elements of NephCure’s brand evolution include:
New Logo: The new NephCure logo represents community and togetherness. Featuring a modern spiral of cascading lines that converge together to form a kidney, it demonstrates NephCure’s focus on facilitating connections within the RKD community. This shape also doubles as a “spark,” representing NephCure’s position of leadership and innovation in the nephrology field as they work to advance rare kidney disease research toward more effective treatments.
Shortened Name: Through comprehensive research, it was discovered that “NephCure” by and large was the most prevalent name people used to refer to the organization. Since there was high awareness and equity in this specific part of the former name, they decided to officially shorten it to simply “NephCure,” while also developing a supporting tagline to further clarify their mission.
New Tagline: “For rare kidney disease” reflects NephCure’s distinct focus on RKD, as well as their passion and focus to continuously strive to find a cure.
New Vision Statement: “A world where all who are affected by rare, protein-spilling kidney disease are connected to new and better treatments — and one day, a cure.”
New Mission Statement: “To empower people with rare, protein-spilling kidney disease to take charge of their health, while leading the revolution in research, new treatments, and care.”
For the second phase of NephCure’s rebranding work, the organization plans to update its website, NephCure.org, to reflect the new brand updates that have gone into effect today. NephCure will continue to update its community on the status of this website redesign.
To learn more about NephCure’s new visual and written branding, visit brand.nephcure.org.
About NephCure:
NephCure’s mission is to empower people with rare, protein-spilling kidney disease to take charge of their health, while leading the revolution in research, new treatments, and care. Founded in 2000 by a group of committed patient parents, NephCure has invested more than $40 million in kidney disease research and helped create a landscape where there are now new treatments and more than 60 interventional drug trials for rare kidney diseases. NephCure is a U.S. tax exempt 501(c)(3) public charity.
###



 Let the games begin! NephCure has created the Kidney Month Bingo Challenge
Let the games begin! NephCure has created the Kidney Month Bingo Challenge