When I was 17, I was diagnosed with kidney disease called FSGS. This was 1 year after my little brother had been diagnosed with the same thing. While my brother was able to be managed on medication, mine was more malignant. It was not explained well to
me at the time so it was hard for my family and I to figure out why it was happening and what I did wrong to cause this. I was placed on heavy steroids and Myfortic. Due to the side effects of the steroids, I had a difficult time during my senior year of high
 school.
school.
Once I graduated, I went to Texas Tech to try and enter their fast-track medical school program. This was when I started to experience kidney failure. I was in denial about my symptoms and was thinking the swelling and fatigue were side effects of the
medications. I was so swollen my skin would literally tear open and streams of water would come out. It was only when my mom and sister begged me to go to the college clinic that I went and was told it was a miracle I was still alive and that I needed to go to the emergency room immediately. I flew back home and when my parents saw how swollen I was they bursted into tears. I was taken to the ER where they placed an emergency catheter to begin dialysis. I was told I had 40 liters of extra fluid in my body. I was now on hemodialysis 3 times a week for a few months before transferring to peritoneal dialysis. I immediately re-enrolled at the University of Houston. Between the adjustments to my life, school, and work, I was not keeping up with my treatments correctly, which led me to having a seizure in my dorm room, falling and hitting my head on the corner of my desk.
 Luckily, I was rooming with my little brother so he was able to call an ambulance immediately. I was in a coma and the doctors said they had no idea if or when I would wake up. After three days, I woke up from the coma. Between this and college I was placed on probation at the University of Houston. I then transferred to Texas Southern University to finish my last year of college. After graduating, I was accepted into the Texas Southern University college of pharmacy where I also received my kidney transplant after 5 years on dialysis. I was told to withdrawal from school due to being behind in courses and needing to recover due to my health. But, fortunately my body healed quicker than expected and I was able to catch up and finish the semester on the Dean’s list.
Luckily, I was rooming with my little brother so he was able to call an ambulance immediately. I was in a coma and the doctors said they had no idea if or when I would wake up. After three days, I woke up from the coma. Between this and college I was placed on probation at the University of Houston. I then transferred to Texas Southern University to finish my last year of college. After graduating, I was accepted into the Texas Southern University college of pharmacy where I also received my kidney transplant after 5 years on dialysis. I was told to withdrawal from school due to being behind in courses and needing to recover due to my health. But, fortunately my body healed quicker than expected and I was able to catch up and finish the semester on the Dean’s list. 
A couple of years later my little brother progressed to kidney failure and then had to begin hemodialysis. He was not living with my parents at this time so between my mom and I, we would take him to his treatments. He then changed to peritoneal dialysis. After a few months and issues with his dialysis treatment he passed away in his apartment. I still feel guilty about not being able to provide him with more support during that time. This is why shedding light on the effects of rare kidney disease is a passion, not a pastime. I want to help others who were not as fortunate and blessed as I was to make it through so many obstacles and hardships, in whatever way possible.
— Nicholas Ariyo, 4th year Pharm.D candidate at TSU College of Pharmacy



 On December 9, 2023, researchers, doctors, regulators, and patients from across the globe met in Washington, D.C. at the Proteinuria and GFR as Clinical Trial Endpoints in Focal Segmental Glomerulosclerosis (PARASOL) Project to discuss developing biostatistical models that could help with the design of clinical trials and aid subsequent regulatory approval of new treatments for FSGS.
On December 9, 2023, researchers, doctors, regulators, and patients from across the globe met in Washington, D.C. at the Proteinuria and GFR as Clinical Trial Endpoints in Focal Segmental Glomerulosclerosis (PARASOL) Project to discuss developing biostatistical models that could help with the design of clinical trials and aid subsequent regulatory approval of new treatments for FSGS.






 About my fourth year with my disease, my doctor asked me if I wanted to try a different medication to get me off of Prednisone completely. That’s when she introduced Tacrolimus. I am taking 2mg of Tacrolimus and going strong.
About my fourth year with my disease, my doctor asked me if I wanted to try a different medication to get me off of Prednisone completely. That’s when she introduced Tacrolimus. I am taking 2mg of Tacrolimus and going strong.

 The nephrologist quickly reviewed historical labs and ordered new ones. He found that there had been a long slow trend of protein spillage. He quickly asked Anthony to get a biopsy so they could identify exactly what was going on.
The nephrologist quickly reviewed historical labs and ordered new ones. He found that there had been a long slow trend of protein spillage. He quickly asked Anthony to get a biopsy so they could identify exactly what was going on.



 irregular heartbeat. His blood pressure at the time was 234/176 when recorded from his right arm, yet
irregular heartbeat. His blood pressure at the time was 234/176 when recorded from his right arm, yet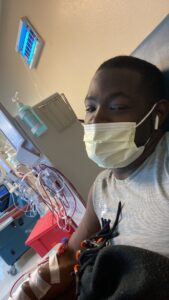 started affecting
started affecting 

