Goldfinch Bio is a biotechnology company based in Cambridge, MA which focuses on delivering disease-modifying precision medicines that bring hope and renewed quality of life to people living with kidney diseases. We recently spoke with Goldfinch Bio’s Chief Medical Officer, Ed Tucker, MD, who explains more about their Phase 2 clinical research trial for GFB-887, TRACTION-2, which is currently enrolling patients.

What patients are you hoping to enroll for this trial?
In the TRACTION-2 study that is currently enrolling, we are looking for patients with focal segmental glomerulosclerosis (FSGS) and treatment resistant minimal change disease (TR-MCD) who are between the ages of 18 and 75 years old, have a UPCR greater than or equal to 1.0 g/g, and an eGFR greater than or equal to 30 mL/min/1.73 m2. Other inclusion and exclusion criteria will apply; please consult your healthcare provider for additional details.
Why is FSGS and TR-MCD your target population?
In the United States, there are currently no approved therapies for FSGS or MCD. Our treatment, GFB-887, was designed specifically for diseases like FSGS and TR-MCD because it targets and protects the podocytes that are damaged and lost in the disease development and progression. Other diseases involving podocyte injury/loss may be examined in the future as well.
If someone enrolled in your Phase 2 study, what is the time commitment?
After a patient has been evaluated (for up to 6 weeks) for eligibility in the study they will begin to receive the treatment and will continue for up to a total of 17 visits over approximately 26 weeks. Some of these visits are done via a phone call. After completion of the full study, patients will have the option to enroll in another “extension” study where they will continue to receive treatment for up to three years. An extension study is where all patients receive GFB-887 and allows for doctors to observe the effects of the therapy over a longer period of time.
How many trial sites are there available? Where are these sites located?
Currently, there are 76 locations throughout the United States where patients may participate in the study. Locations of these sites may be found here.
What type of treatment is GFB-887? And what makes this treatment different from other ones being tested in clinical trials?
GFB-887 is a tablet and is taken by mouth with water once a day. It differs from other medications in that it was created specifically to target and protect podocytes in the kidney to prevent damage and loss. No other therapies being tested for FSGS and TR-MCD work the same way that GFB-887 does on the kidneys.
How is precision medicine integrated in this clinical trial?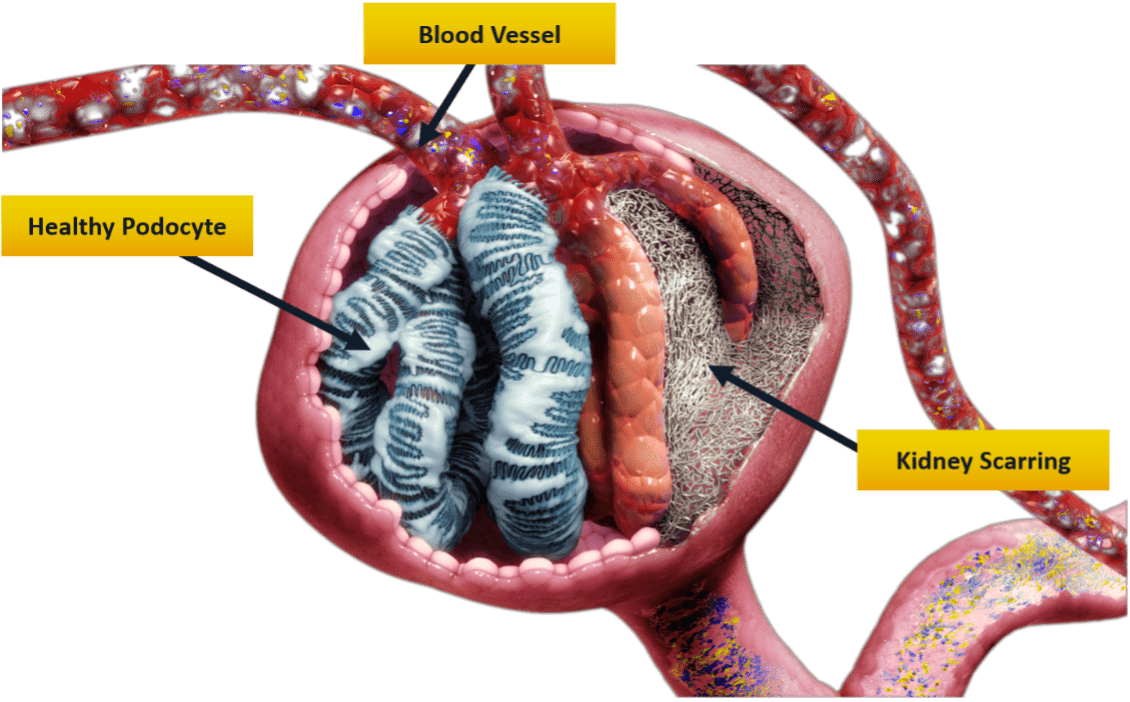
As this is a phase 2 trial, we are looking at different markers (including testing for genetic) for response to GFB-887, both before and after treatment has begun. Hopefully, we will be able to better integrate these findings into future studies and potentially improve outcomes for patients.
Is there flexibility where lab samples can be collected for the participant? Is there an option for home care visits to collect these samples?
Yes, patients have the flexibility to schedule follow-up visits within a few days before and after the expected date. Furthermore, if conditions outside the control of the patient change (Covid for example), or if the patient is unable to travel due to health limitations, the study site will work with the patient on scheduling and potentially offer telemedicine options for select visits. Additionally, your study site may be able to assist with travel services to and from office visits.
What medicines can participants remain on while in the TRACTION-2 trial?
Some medications, including calcineurin inhibiters (CNIs; cyclosporine for example), will need to be discontinued. Others, including angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB) should be continued, while various other drugs may be continued if on a stable dose. Please consult your healthcare provider for additional details.
Does this trial have a placebo comparison?
Yes. The placebo is used because comparing results in study participants who receive GFB-887 with results in participants who receive placebo is the best way to explore how well GFB-887 works and how safe it is. 66% of patients will receive GFB-887 (33% will receive placebo), but all patients will have the option to join an extension study where all patients will be offered GFB-887 for up to three years.
What will happen to the results of this clinical trial? Can the participant stay on the medicine if it works?
The results of this study will help guide the additional exploration of GFB-887 in these diseases and potentially others. It is one of many steps required before the FDA will approve a drug for broader availability. Currently, participants, once completing the initial study, have the option to participate in another “extension” study for up to three years. If a patient was taking placebo during the initial trial, they will receive GFB-887 in the “extension” study.
Do you have plans for expanding into a pediatric trial?

The current trial does not allow for patients under the age of 18, but we hope to offer additional options for younger patients in the future.
Will the patients be paid for participating in the trial?
Patients may be eligible to receive compensation for time and reasonable out-of-pocket expenses related to taking part in this trial. For more information, please talk to your trial doctor and trial staff.
Goldfinch Bio’s TRACTION-2 clinical research trial is evaluating an investigational precision medicine, GFB-887, for the potential treatment of TRMCD and FSGS. The purpose of the trial is to determine if GFB-887 is safe and may help people who have high levels of protein in their urine due to kidney diseases caused by podocyte injury.
To learn more about the TRACTION-2 clinical trial, click here. To see a full list of clinical research opportunities and find the right trial for you, visit KidneyHealthGateway.com.
This article was developed in partnership with Goldfinch Bio, Inc.