 Dr. Jeffrey Kopp is a physician and researcher who focuses on FSGS and related diseases. He currently leads a group in the kidney disease section (officially called the National Institute of Diabetes and Digestive and Kidney Diseases, or NIDDK) of the National Institutes of Health (NIH). Dr. Kopp is also working on a new clinical trial for FSGS, MCD, and MN patients at the NIH headquarters near Washington D.C. We had the awesome pleasure of sitting down and catching up with Dr. Kopp about his fascinating job and new clinical trial. Keep reading to learn more, and read about some of his other research projects here.
Dr. Jeffrey Kopp is a physician and researcher who focuses on FSGS and related diseases. He currently leads a group in the kidney disease section (officially called the National Institute of Diabetes and Digestive and Kidney Diseases, or NIDDK) of the National Institutes of Health (NIH). Dr. Kopp is also working on a new clinical trial for FSGS, MCD, and MN patients at the NIH headquarters near Washington D.C. We had the awesome pleasure of sitting down and catching up with Dr. Kopp about his fascinating job and new clinical trial. Keep reading to learn more, and read about some of his other research projects here.
Interview highlights:
- Dr. Kopp works at the National Institute of Health’s kidney branch, where he studies glomerular diseases such as FSGS and MCD. He also serves as Captain for the United States Public Health Service, and has been deployed to help with medical care during natural disasters.
- Dr. Kopp is leading a new clinical trial for FSGS, MCD, and MN patients at the NIH studying a compound called ManNAc as a treatment option.
- ManNAc is a sugar that occurs naturally in your body. Another researcher at the NIH found that mice without ManNAc developed MCD, and adding ManNAc to their diet was helpful in treating it. Therefore, it may be effective at treating MCD, FSGS, and MN in humans (Dr. Kopp describes the full mechanism below—make sure you read the article!)
- This study requires people to stay at the NIH for 11 days total, but it can be split up into 2 trips. Luckily, there is a lot to do to pass free time you may have at the NIH, including movie marathons, exercise programs, an art gallery, and an in-house business center.
- Learn more about taking part in the study by clicking here or contacting Emily Brede, RN at emily.brede@nih.gov
Full interview:
NKI: What is your job at the NIDDK?

Dr. Kopp: I am fortunate to lead a translational research group at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), which is part of the National Institutes of Health. Our mission is to develop a better understanding of the disease mechanisms responsible for focal segmental glomerulosclerosis (FSGS) and to develop more effective and less toxic therapies.
I also serve in the United States Public Health Service, with a rank of Captain. My primary mission at NIH is to carry out basic and clinical research in FSGS. I also deploy for public health emergencies, such as natural disasters. Thus, I participated in the medical response to Hurricanes Katrina and Ike.
SIDE NOTE: What is NIH?
Dr. Kopp: The NIH is a federal biomedical research facility located in Bethesda, MD. The campus includes a 240-bed Clinical Research Center and extensive outpatient clinics. Every patient who comes to NIH participates in a research protocol. Some protocols involve novel treatments and other protocols involve giving samples for research. NIH physicians may give advice about standard therapies that can be used. There are no charges for any medical care provided by the NIH Clinical Center.
NKI: What do you enjoy about CKD research?
Dr. Kopp: CKD, and particularly glomerular diseases (such as FSGS), are incompletely understood, and the available therapies are not ideal. I like the challenge of understanding and treating these diseases, and most of all I like the opportunity to improve the lives of patients with these conditions.
NKI: The newest clinical trial for FSGS, MCD, and MN patients at the NIH is looking at MaNAc as a treatment option. Why did you decide to study MaNAc?
Dr. Kopp: A colleague at NIH developed mice unable to make ManNac. She found that these mice developed glomerular disease soon after birth. This disease resembled a human glomerular disease, minimal change disease. Providing extra ManNAc orally to the mice cured the kidney disease. This prompted the question: can we use ManNAc to induce remissions in our patients?
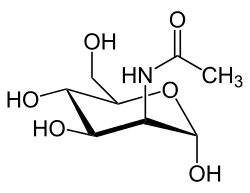
NKI: What is ManNAc?
Dr. Kopp: Perhaps the word sounds to you like manna, the food the Israelites found in the desert and that helped sustain them. There is a tree in Europe that exudes a sweet white resin, similar to the sap of the sugar maple, and people who knew the Bible story called the tree the manna tree. A chemist found a distinctive and novel sugar in the manna resin, and he called the new sugar “mannose”.
NKI: Does ManNAc occur naturally in the body? Is it found in food?
Dr. Kopp: ManNAc is a natural product and essential for good health. Our food does not contain much ManNAc. Our bodies make ManNAc, which is converted in our cells to mannose. This in turn is converted to sialic acid, which is put on many proteins. All of these are sugars, but they differ from glucose in that they are not related to diabetes and they are present in very small amounts, so that they do not add calories in the diet.
NKI: What is the reason for believing that ManNAc might be useful in treating glomerular diseases?
 Dr. Kopp: Podocytes are cells on the outside of the kidney glomeruli and serve to prevent plasma proteins from leaking into the urinary space. Many patients with glomerular diseases have lost sialic acid from the proteins on the podocyte. We think that providing extra ManNAc might promote the return of sialic acid to podocyte proteins and that this might improve podocyte function. We see some evidence in mouse models of FSGS that supplemental ManNAc in the diet helps treat these mice.
Dr. Kopp: Podocytes are cells on the outside of the kidney glomeruli and serve to prevent plasma proteins from leaking into the urinary space. Many patients with glomerular diseases have lost sialic acid from the proteins on the podocyte. We think that providing extra ManNAc might promote the return of sialic acid to podocyte proteins and that this might improve podocyte function. We see some evidence in mouse models of FSGS that supplemental ManNAc in the diet helps treat these mice.
NKI: What is involved for patients in this study?
Dr. Kopp: Patients will provide their medical records for review by the NIDDK team. We also review the kidney biopsy materials from past kidney biopsy. No kidney biopsy is done as part of this study. If patients appear to qualify for the study, they will come to NIH for an outpatient visit for evaluation and to discuss study participation.
NKI: Is travel to NIH paid for?
Dr. Kopp: Travel to NIH can be arranged and provided by NIH. If overnight accommodation is needed, NIH can provide this also.
NKI: Why are patients required to stay at the NIH during this study?

Dr. Kopp: The study requires being an inpatient for 11 days, either as a single stay or as two stays of five and six days. The reason for the inpatient stay is allow frequent sampling of blood and urine and for safety, to be sure there are no side effects.
NKI: What can patients do with any “free time” during the study? How much free time do you expect patients to have?
Dr. Kopp: During the first five days, there are frequent time points for sample collection. During the second six days, samples are needed at 8 am and 8 pm. There is extensive free time that patients can use as they like.
There are many activities that can help pass the time at NIH
• Patient Computers combination television and computer (with Internet access) at most patients’ bedsides to provide access to games, web browsing, and personal e-mail via the Internet
• Patient Library has more than more than 5,000 books, including a selection of current best-sellers, reference, foreign language, large-print, picture, and audio books
• Clinical Center’s Fine Art Program has more than 2,000 works of art. Most artwork remains on permanent display throughout the hospital, but there are six galleries on the first floor that change every eight weeks. A walking tour is available to assist patients, caregivers and visitors in their enjoyment of the artwork on display.
•Recreation Therapy programs include:
o Arts and crafts
o Music
o Games and sports
o Social events
o Exercise
o A large selection of DVD movies
o Instruction in coping skills such as relaxation, enhanced communication, and stress management
• Spiritual Care Department offers Catholic, Jewish, Islamic, and Protestant services in the interfaith chapel
• Business Center has four PCs and four MACs (all with Internet connection) as well as a combined printer/copier/FAX and telephones are available.
NKI: Who can participate in the ManNAc study?
Dr. Kopp: We are recruiting adults (age ≥18 years) with a primary glomerular disease, including minimal change disease, FSGS, and membranous nephropathy, and with nephrotic range proteinuria (urine protein/creatinine ratio > 2 g/g).
Exclusion criteria include having diabetes mellitus and receiving pulse therapies, such as rituximab. Monetary compensation is provided.
NKI: How do I get more information about the study?
Dr. Kopp: The study, like all clinical research studies, is described at clinicaltrials.gov.
You also contact the study research nurse, Emily Brede, RN at Emily.brede@nih.gov


