What is sparsentan and how is it taken?
Sparsentan is a novel Dual Endothelin Angiotensin Receptor Antagonist (DEARA) being studied as a potential treatment option for immunoglobulin A (IgA) nephropathy and focal segmental glomerulosclerosis (FSGS). In clinical trials, sparsentan is being given as a tablet or age-appropriate liquid taken by mouth.
What is the next step in the approval process?
The U.S. Food and Drug Administration (FDA) has confirmed the Prescription Drug User Fee Act (PDUFA) target action date for sparsentan in IgA nephropathy is February 17, 2023.
Can you describe the patients in the DUPLEX and PROT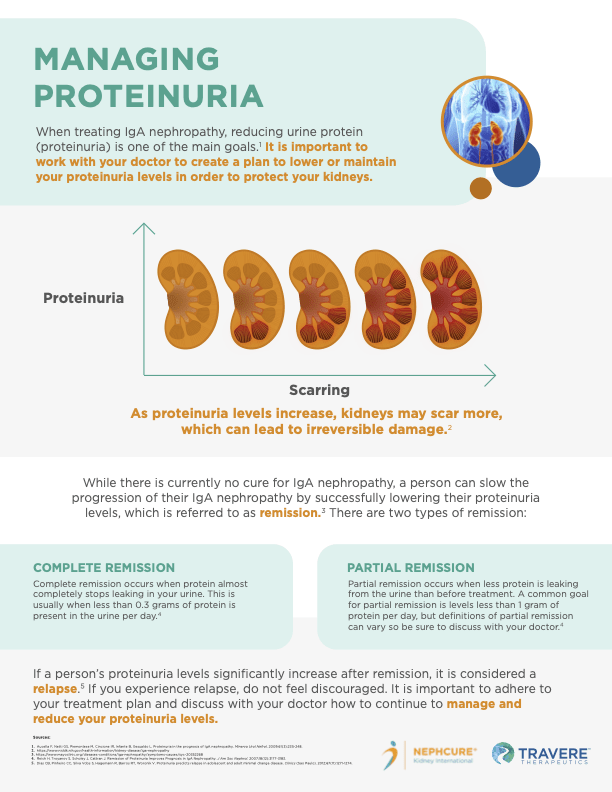 ECT studies?
ECT studies?
DUPLEX and PROTECT are ongoing Phase 3 clinical trials studying the efficacy and safety of sparsentan. The DUPLEX study includes 371 patients, ages 8 to 75 years, with primary FSGS. The PROTECT study includes 404 patients, ages 18 years or older, with IgA nephropathy and persistent proteinuria despite angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) therapy.
Can you explain to our patients how sparsentan reduces proteinuria?
The amount of protein found in the urine (called proteinuria) is seen as a marker of kidney function. Lowering proteinuria levels is associated with better kidney health outcomes. Pre-clinical data have shown that blocking both endothelin type A and angiotensin II type 1 pathways, in forms of rare chronic kidney disease, reduces proteinuria. In the PROTECT study, after 36 weeks of treatment, patients receiving sparsentan experienced a greater than threefold reduction of proteinuria from baseline (49.8 percent) compared to the active control irbesartan (15.1 percent).
How is sparsentan different from other treatment options?
Sparsentan is different from other medications because it is non-immunosuppressive and it is dual-acting (combines two mechanisms of action into one molecule). It selectively blocks the action of two important mediators of progression to kidney failure (endothelin type A and angiotensin II type 1) at their receptors.
Can you give us an update about the use of sparsentan for FSGS patients?
Travere Therapeutics anticipates having topline data from the DUPLEX study, including full two-year estimated glomerular filtration (eGFR) data, in the first half of 2023. The company is planning to pursue traditional approval of sparsentan for FSGS in the United States in 2023 and Conditional Marketing Authorization in Europe.
What’s next for Travere Therapeutics?
Travere Therapeutics is dedicated to working with the rare disease community to identify, develop, and deliver life-changing therapies. In addition to the development efforts for sparsentan in IgA nephropathy and FSGS, the company is advancing pegtibatinase for the treatment of classical homocystinuria, a genetic metabolic disorder that can cause life-threatening thrombotic events. In addition, early research efforts include partnering with leaders in patient advocacy and government research to identify potential therapeutics for Alagille syndrome, a rare genetic disorder that can affect the liver, heart, skeleton, eyes and kidneys.
Where can patients with more questions go to find answers?
For more information, patients should talk to their healthcare provider and can visit travere.com, rkdandme.com, navigateigan.com, or lowerproteinuria.com.